by S. Pagani (MOX, Politecnico di Milano) and A. Frontera (MD, Ph.D., Head of electrophysiology unit, IRCCS Humanitas Research Hospital).
Atrial fibrillation (AF) is the cardiac rhythm disorder with the highest worldwide incidence. Besides impacting the patient’s quality of life, AF increases stroke risk and leads to several heart complications. AF is a disease characterized by a progression of structural, functional, and systemic dysfunctionalities. When untreated, AF advances rapidly with episodes of increased duration and frequency, impinging on the preexisting dysfunctionalities (“AF begets AF”). Therefore, the main challenges in patient care are early diagnosis and personalized treatments aimed at controlling and slowing the progression.
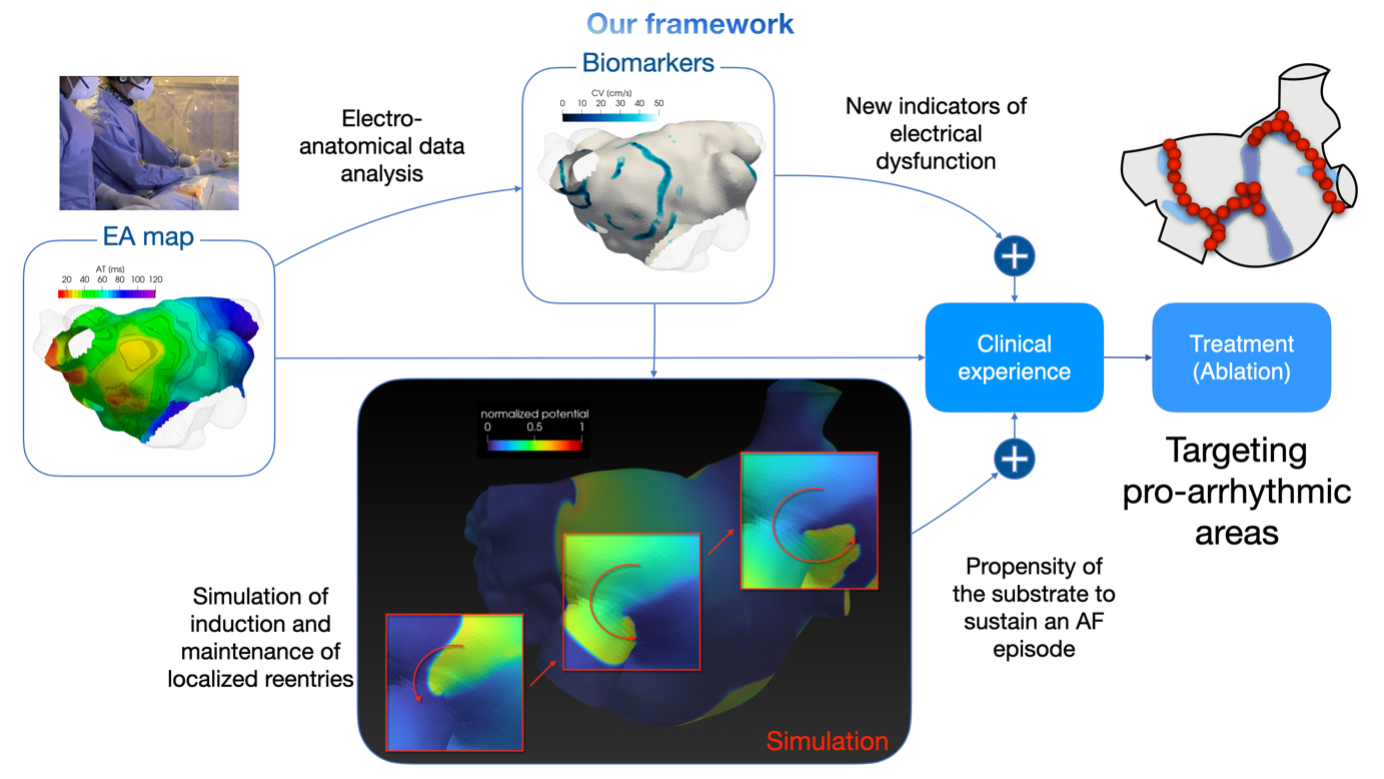
The electrophysiological study followed by trans-catheter ablation is one of the gold standard treatments for AF. First, the electrophysiologist collects electro-anatomical (EA) data, that is, the set of electrical (electrograms) and geometric (location of the sensor during the measurement) information. Then, radiofrequency ablation targets portions of tissue inclined to sustain an arrhythmic episode. Linking electro-anatomical data to arrhythmic propensity is extremely challenging: it is specific to each patient and thus requires tailoring treatment. For this reason, standard ablation approaches based only on anatomical features remain suboptimal.
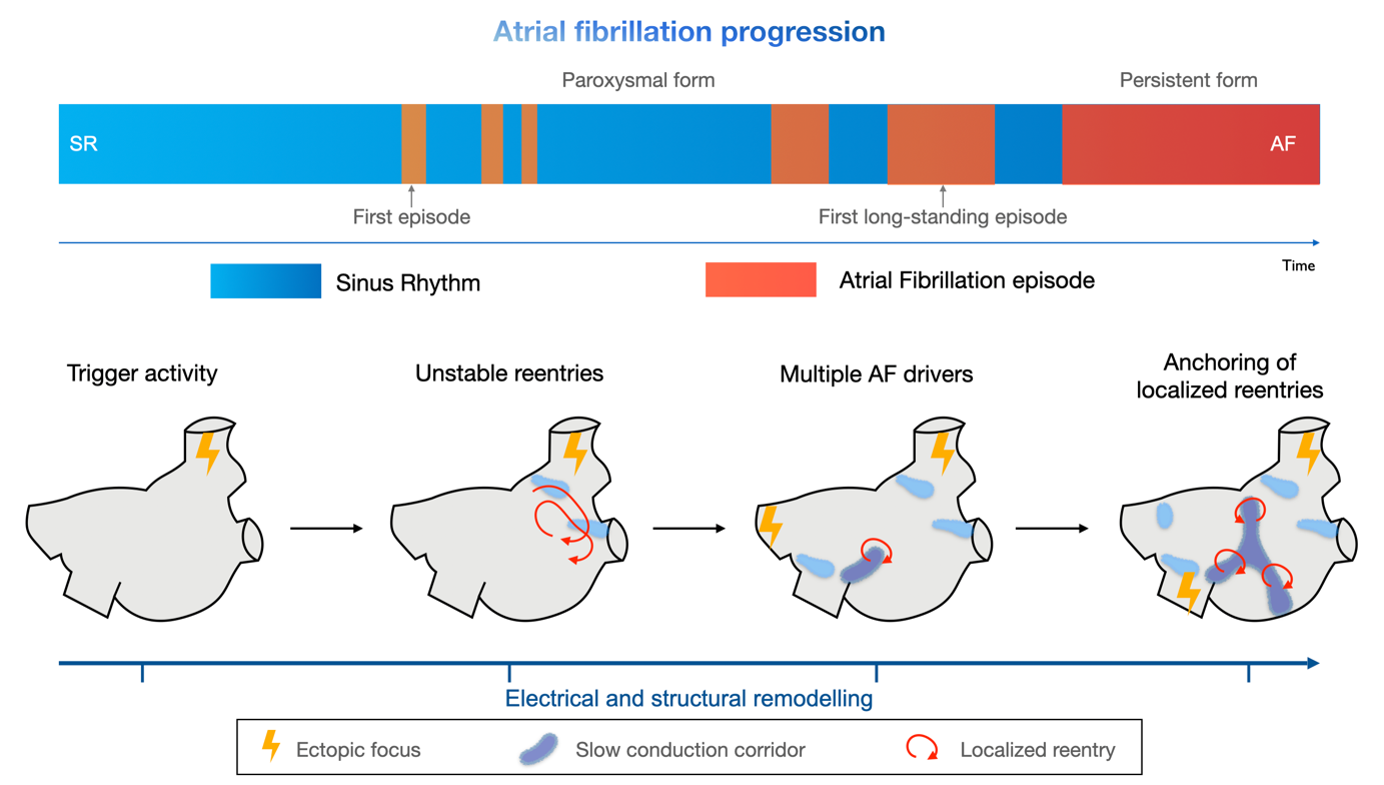
A success story. Our research activity focused on identifying quantitative indicators of electrical dysfunctionality through numerical post-processing of electro-anatomical maps [1]. Specifically, we found that an increase in slow-conduction corridors and pivot points characterizes disease progression: they stratify patients with episodes lasting less than seven days from those with a persistent form (see Figure 1). Our numerical simulations confirm the link between these indicators and arrhythmic propensity: an atrial substrate formed by severe slow conduction and pivot points enables localized reentries characterizing an AF episode to be self-sustained for longer than mild substrate characteristics [2].
Main clinical outcome. Our research let us connect arrhythmic propensity with electrophysiological indicators obtained by processing electro-anatomical maps. This approach provides a new direction for tailoring the ablation strategy based on the patient’s individual electrophysiological properties (see graphical representation of the whole process in Figure 2).
Clinical comment by A. Frontera, MD, Ph.D. “Through this research in collaboration with MOX, I further innovate my approach to each new patient. During procedures, we perform an online analysis of endocavitary signals [3,4]: the focus is the identication of areas of slow conduction and pivot points. I believe this might help to tailor the treatment for each patient, ensuring recovery of sinus rhythm with low recurrences. Our results are also stimulating the debate in the electrophysiology community, strengthening electrophysiological characterization at the center of AF patient care.”
Bibliography
[1] A. Frontera, S. Pagani, L.R. Limite, A. Peirone, F. Fioravanti, B. Enache, J.C. Silva, K. Vlachos, C. Meyer, G. Montesano, A. Manzoni, L. Dede’, A. Quarteroni, D.G. Lațcu, P. Rossi, P. Della Bella. “Slow conduction corridors and pivot sites characterize the electrical remodeling in atrial fibrillation”. JACC Clinical Electrophysiology, In press, 2022.
[2] S. Pagani, L. Dede’, A. Frontera, M. Salvador, L.R. Limite, A. Manzoni, F. Lipartiti, G. Tsitsinakis, A Hadjis, P Della Bella, A Quarteroni. “A computational study of the electrophysiological substrate in patients suffering from atrial fibrillation”. Frontiers in Physiology, 12:927, 2021.
[3] A. Frontera, L.R. Limite, S. Pagani, A. Hadjis, M. Cireddu, S. Sala, et al. “Characterization of cardiac electrogram signals in atrial arrhythmias”. Minerva Cardiology and Angiology, 69(1):70–80, 2021.
[4] A. Frontera, L.R. Limite, S. Pagani, et al. “Electrogram fractionation during sinus rhythm occurs in normal voltage atrial tissue in patients with atrial fibrillation”. Pacing and Clinical Electrophysiology, 45(2): 219-228, 2022.