A new MOX Report entitled “A novel mathematical and computational framework of amyloid-beta triggered seizure dynamics in Alzheimer’s disease” by Leimer Saglio, C. B.; Corti, M.; Pagani, S.; Antonietti, P. F. has appeared in the MOX Report Collection.
Check it out here: https://www.mate.polimi.it/biblioteca/add/qmox/68-2025.pdf
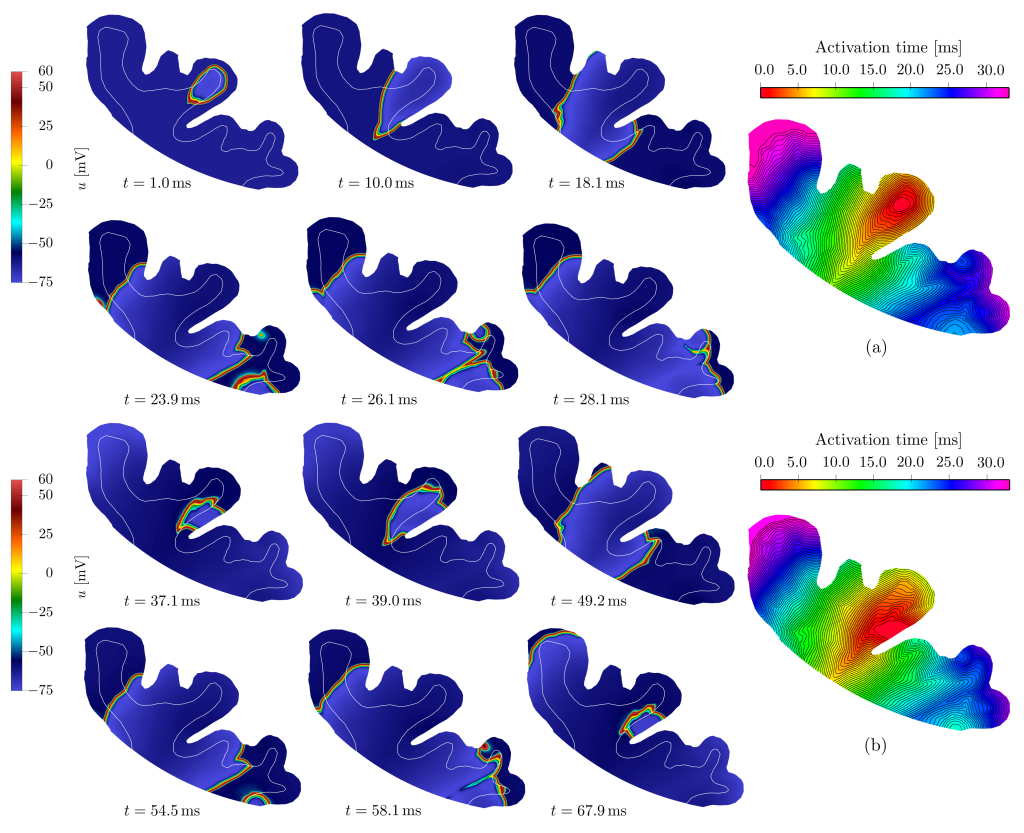
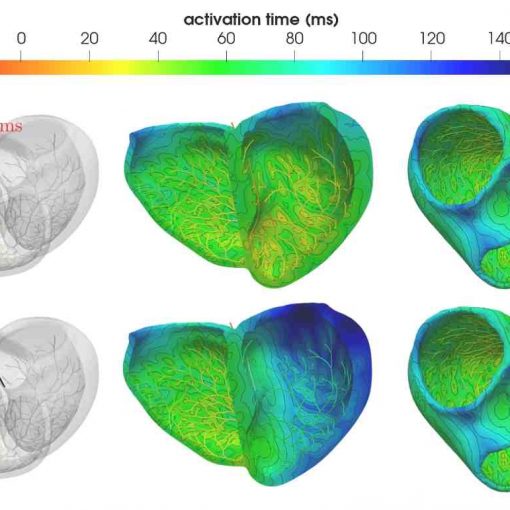
Abstract: The association of epileptic activity and Alzheimer’s disease (AD) has been increasingly reported in both clinical and experimental studies, suggesting that amyloid-beta accumulation may directly affect neuronal excitability. Capturing these interactions requires a quantitative description that bridges the molecular alterations of AD with the fast electrophysiological dynamics of epilepsy. We introduce a novel mathematical model that extends the Barreto-Cressman ionic formulation by incorporating multiple mechanisms of calcium dysregulation induced by amyloid-beta, including formation of calcium-permeable pores, overactivation of voltage-gated calcium channels, and suppression of calcium-sensitive potassium currents. The resulting ionic model is coupled with the monodomain equation and discretized using a p-adaptive discontinuous Galerkin method on polytopal meshes, providing an effective balance between efficiency and accuracy in captur! ing the s harp spatiotemporal electrical wavefronts associated with epileptiform discharges. Numerical simulations performed on idealized and realistic brain geometries demonstrate that progressive amyloid-beta accumulation leads to severe alterations in calcium homeostasis, increased neuronal hyperexcitability, and pathological seizure propagation. Specifically, high amyloid-beta concentrations produce secondary epileptogenic sources and spatially heterogeneous wavefronts, indicating that biochemical inhomogeneities play a critical role in shaping seizure dynamics. These results illustrate how multiscale modeling provides new mechanistic insights into the interplay between neurodegeneration and epilepsy in Alzheimer’s disease.